- Home
- Assessments
- Bioregional Assessment Program
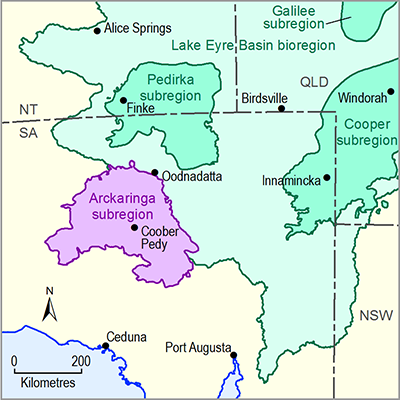
- Arckaringa subregion
- 1.1 Context statement for the Arckaringa subregion
- 1.1.7 Ecology
- 1.1.7.3 Aquatic species and communities
The aquatic ecological systems of the Arckaringa subregion can be broadly grouped into the groundwater-dependent GAB springs, the surface water dependent aquatic ecosystems of the western LEB catchments, and the surface water-dependent ecosystems to the west of the LEB. The following sections describe each of these groupings, firstly in terms of the broader aquatic ecosystems and habitats and secondly in relation to the species and communities therein. The aquatic biota of isolated endorheic systems and salt lakes and their catchments are very poorly understood and in the Arckaringa subregion have not been studied.
Because the GAB springs are decoupled from regional climatic rainfall changes, they provide evolutionary refugia for relict and short-range endemic species that have limited capacity to persist or disperse without permanent water. The GAB spring ecosystems are therefore highly vulnerable because (for the most part) their biota cannot re-populate springs once they become locally extinct. Perennial waterholes in arid zone river systems provide ecological refuges for obligate aquatic species and are responsive to local and regional climatic conditions (Davis et al., 2013). Within the Arckaringa subregion there are no known permanent surface water-driven waterholes, however Algebuckina Waterhole, 2 km downstream in the Neales catchment is not known to have dried out in living memory. However, other semi-permanent waterholes and GAB springs also provide various refuge roles that sustain surface water biota (McNeil et al., 2011a). Populations of aquatic biota have distinct assemblages and distributions at different scales (e.g. spring vent, spring group, waterhole, and catchment) depending on their dispersal mechanisms and capacity to withstand conditions within those environments. The relationship between hydrological connectivity and dispersal between catchments can only be determined by genetic studies and is a priority for further research (Davis et al., 2013). The long term persistence of the species and communities of the Arckaringa subregion aquatic ecosystems is dependent on maintaining the environmental drivers that support the metapopulation dynamics and requirements of biota through connectivity, refuges, resistance and resilience (Davis et al., 2013). Therefore, the discussion below identifies both the species and communities within the aquatic ecosystems as well as the processes and habitat requirements that are critical to their survival.
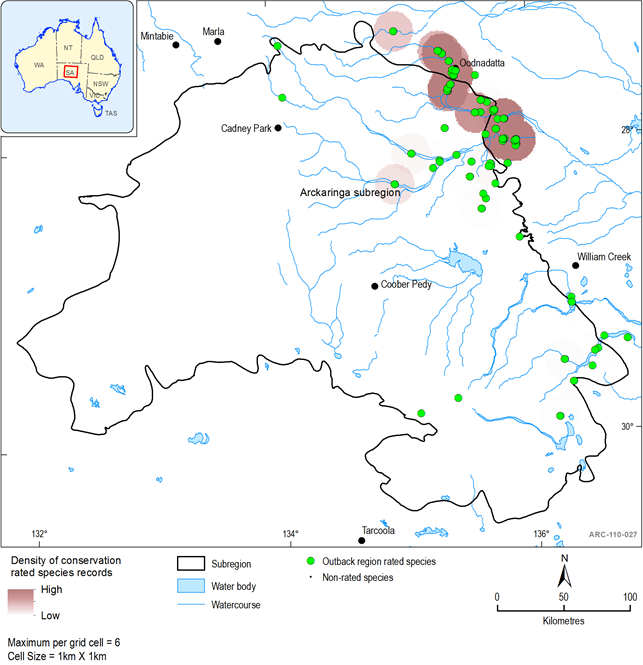
Using data sourced from the Biological Database of South Australia and Atlas of living Australia, the distribution of threatened fish species for the Arckaringa subregion are shown in Figure 40. All records from sites within a 5 km buffer of the subregion are displayed and shown as having a conservation ranking if they have a threatened status of critically endangered, endangered, vulnerable or rare under Commonwealth and State legislation, together with Regional ratings.
1.1.7.3.1 Fish
Thirteen taxa from seven families have been recorded from the subregion. Seven of these are regarded as Rare at the regional level (Table 17). Figure 5 shows the distribution of rated species records within each rating category and the density of rated species records within 1 km2 grid cells. The highest density of fish records are to the south-west of Oodnadatta in the Neales catchment (Figure 40). The only introduced species recorded in the region is Eastern Gambusia (Gambusia holbrooki)
Table 17 List of fish by family with conservation status ratings at the National (EPBC Act), South Australian (SA NPWS Act) or regional level (Outback NRM Region) recorded within the Arckaringa subregion
aintroduced species
1.1.7.3.2 Amphibians
Four taxa from two families of amphibians have been recorded in the subregion however none has any conservation status at the national, state or regional level (Table 18).
Table 18 List of amphibians by family recorded within the Arckaringa subregion
|
Species |
Common Name |
|---|---|
|
Cyclorana mainia |
Main's Frog |
|
Cyclorana platycephalaa |
Water-holding Frog |
|
Litoria rubellaa |
Desert Tree Frog |
|
Neobatrachus sudellia |
Sudell's Frog |
aWetland, drainage-line or floodplain dependent taxa are indicated
1.1.7.3.3 Groundwater-dependent aquatic ecosystems
Function of Great Artesian Basin springs
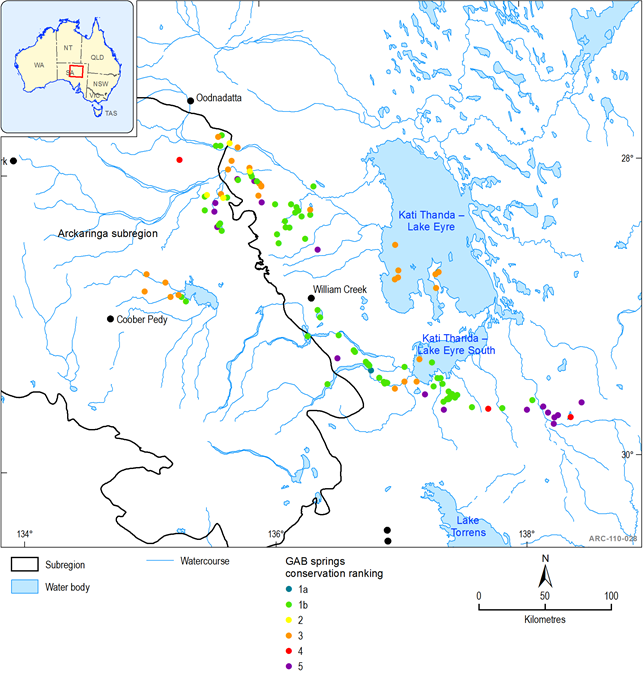
Spring ecosystems dependent on discharge from the GAB are rated as nationally endangered and are protected under the EPBC Act. GAB springs from the Lake Eyre supergroup of springs are also listed in the directory of important wetlands in Australia and occur within and to the east and south-east of the Arckaringa subregion (Figure 41). The hydrogeology of the GAB relating to these springs is discussed in section1.1.4
Gotch (2013a) developed a spring nomenclature to provide a common language to describe GAB springs in SA (Table 19) based on previous work by Ponder (1986) and Fatchen and Fatchen (1993). A similar nomenclature is adopted in Queensland based on Fensham and Fairfax (2003), however the latter used distance definitions to separate between spring groups and between spring complexes. Gotch (2013a) surveyed 4516 springs vents in 103 spring groups in SA (Table 22) with 3793 vents found in the Lake Eyre supergroup. 85 spring complexes are entirely active, 17 entirely inactive, and the remaining 14 contain both active and inactive springs (Fensham et al., 2005).
Table 19 Spring classification hierarchy and definitions
Source: Gotch (2013a, p. 15, Table 2.2)
The term ‘mound spring’ has historically been used to describe GAB springs, however not all springs form the characteristic mound. Green et al. (2013) presents a hierarchical classification for GAB springs in SA consistent with the Australian National Aquatic Ecosystems (ANAE) classification framework (AETG 2012a), incorporating the national attributes at the ‘higher levels’. The ‘lower levels’ provide greater differentiation for GAB springs, with attributes for hydraulic environment (artesian or non-artesian), structural linkage to aquifer source and seven surface morphology types (Table 9) (Green et al., 2013). Carbonate mounds are common throughout the Lake Eyre supergroup, but there are also a significant number of sand mounds and peat and fen bogs (Green et al., 2013).
Table 20 Different surface morphology types for artesian GAB Springs
Source: Green et al. (2013, p. 20)
The Gotch (2013a) survey recorded vent position and elevation to a very high degree of accuracy as well as other baseline information on the condition and ecological values such as flow status, water chemistry, stock damage, and species inventory. Some spring groups remain unsurveyed or surveyed to a lower level of accuracy. Elevation of the spring vent is a key attribute as it can be subtracted from the source aquifer pressure to determine the groundwater pressure above the spring surface (Green and Berens, 2013). The pressure above spring surface is an indicator of the vulnerability of spring discharge to reduction in groundwater level, with excess head values of five metres or less indicative of springs at very high risk of ceasing to flow due to reductions in groundwater pressure (Green et al., 2013). The pressure above spring surface has been calculated for the majority of surveyed GAB springs in the artesian zone in SA based on the potentiometric map for the J-K aquifer (see Green et al., 2013 pp. 29-35).
The area of the wetland has been shown to vary over time both between seasons, mainly driven by the phenology of the dominant vegetation species, and over longer time periods, mainly in response to rainfall variation (White et al., 2013). Some vents within a spring group may be permanently connected, either by discharging to the same pool, by overland flow or by saturated soil conditions. Because of intra- and inter-annual variation (White et al., 2013), vents within a spring group that are not permanently connected may become connected seasonally or ephemerally. Within a spring wetland, there is variation in water chemistry and soil conditions which drives variation in plant species composition (Clarke et al., 2013) and habitats. The flow rate of a spring is one of the main determinants of the total wetland area, the connectivity of the wetland system, the water and soil chemistry (Green et al., 2013), and range of vegetation and habitats (Clarke et al., 2013) present within a wetland. Therefore, the ecological values of GAB springs, including those with a relatively high pressure above the spring vent, can be impacted by relatively small reductions in pressure and hence flow.
GAB springs can range from a small soak to large wetland areas supported by numerous vents. Species diversity has been shown to be positively correlated with the number of vents in a spring group (Ponder 1986; Clarke et al., 2013). Vents commonly discharge in a pool that spills over to the tail wetland. Because the conductivity of each spring vent varies, the rate of flow for any given spring vent cannot be determined from excess head alone (Green and Berens, 2013). Spring flow rates are difficult to measure on-ground, however wetland area has been found to provide a surrogate for spring flow rate and can be monitored by remote sensing methods (White and Lewis, 2011, 2012).
The LEB Springs Assessment project has been funded by the Australian Government Department of the Environment through the Office of Water Science to improve the level of knowledge about GAB springs in and near the Arckaringa and Pedirka subregions to inform the Lake Eyre Basin Bioregional Assessment. The focus of this project is on collating existing knowledge about GAB springs and associated wetland habitats, collecting new hydrogeological and ecological knowledge to address knowledge gaps, and assimilating data into hydrogeological and eco-hydrological modelling. The LEB Springs Assessment is being undertaken by the South Australian Government Department of Environment, Water and Natural Resource and the Queensland Department of Science, Information, Technology, Innovation and the Arts and is scheduled for completion in 2015.
Lake Eyre supergroup of springs
GAB springs in the Lake Eyre supergroup are surrounded by extremely arid environments, effectively existing as aquatic islands within a sea of desert. The isolation of springs and their persistence over hundreds of thousands of years of increasing aridity has contributed to high numbers of relict and short-range endemic species (Davis et al., 2013; Gotch 2013b). Fensham et al. (2010) provides a conservation ranking for spring complexes based on the presence of significant species, particularly endemic species, and condition of the wetland environment (Table 21). The highest rank for an individual spring within a complex is applied to the entire complex (Fensham et al., 2010). Within the Lake Eyre supergroup, Coward Springs complex is ranked 1a, and another 64 spring complexes are ranked 1b (Table 21). The nationally endangered endemic GAB spring plant, the Salt Pipewort (Eriocaulon carsonii), is found at Hermit Hill, North West, Old Finniss, Sulphuric, West Finniss, Gosse spring groups/complexes in the Lake Eyre supergroup (Niejalke field data and Fatchen and Fatchen, 1993 in Fensham et al., 2010). Springs in the Lake Eyre supergroup also support three GAB spring endemic crustaceans, two endemic arachnids, 11 endemic molluscs and one endemic flatworm (listed in Fensham et al., 2005; 2010). There are, however, limitations in identifying ecological values by endemism and species presence as not all springs have been comprehensively surveyed, and some biotic groups have been better surveyed and classified taxonomically than others. A recent survey and genetic analysis of invertebrate fauna of Lake Eyre supergroup springs identified 42 new ‘evolutionarily significant units ’, sixteen of which were restricted to only one spring complex (Guzik and Murphy, 2013). This research indicates it is likely that the diversity of short-range endemic species has probably been substantially under estimated. Guzik and Murphy (2013) identified highly vulnerable spring groups and complexes within the Lake Eyre supergroup based on the presence of evolutionary significant units (Table 22).
Many western GAB springs contain microstromatolites which are poorly understood (Gotch 2013b) but are thought to play an important role in the formation of spring mounds (Keppel et al., 2013). A range of waterbirds have been observed at springs with larger wetlands, including four species listed under international treaties (Badman, 1987, 1985).
Table 21 Conservation ranking criteria for Great Artesian Basin spring complexes
Table 22 GAB spring groups and complexes within and near to Arckaringa subregion with high invertebrate endemism
Source: Guzik and Murphy (2013, Appendix 2, pp. 138-141)
Recent studies of aquatic invertebrate fauna in GAB springs in SA have found there is very little dispersal between springs (Guzik and Murphy, 2013). Genetic studies of plant species disjunct from their main range in coastal Australia found they are genetically distinct from their major coastal populations, having become isolated a long time ago (Clarke et al., 2013). Genetic differences between populations at different GAB spring groups also indicated there is limited dispersal of these flora between spring groups (Clarke et al., 2013). Some GAB springs are located in watercourses, lakes and on floodplains; these are therefore connected to the surface water system and to one another during flow events (McNeil et al., 2012). This connectivity provides opportunities for obligate aquatic biota to disperse between springs, as well as providing refugia for aquatic biota normally found in surface water ecosystems (depending on the water chemistry of the springs). The Desert Goby (Chlamydogobius eremius) is the principal native fish resident within GAB springs of the Arckaringa subregion, however in some springs the Desert Goby has been displaced by the introduced livebearer Gambusia holbrooki (McNeil et al., 2011a, 2012). The connectivity of GAB springs and surface water systems is not formally recorded (see section 1.1.6). Bore drain wetland ecosystems have low ecological values, with only a reduced diversity of GAB spring flora that does not include the unique relict and endemic species found in natural GAB spring wetlands (Fensham and Fairfax, 2003; Gotch 2013b). Bore drain fish communities are typically depauperate and dominated by Gambusia holbrooki (e.g. One Mile, Old Peake, Big Blythe) (McNeil et al., 2012). Some bore drain wetlands are, however, valued by the community, including residents and tourists (Phipps 2008) and some may possibly have been constructed to enhance the flow of pre-existing spring vents that may have held cultural significance (Louise Hercus pers. comm. 2013).
1.1.7.3.4 Non-Great Artesian Basin groundwater-dependent ecosystems
Several non-GAB groundwater-dependent ecosystems (GDE) occur on the eastern side of the Davenport and Denison Ranges; these include mountain block spring systems such as Tarlton Springs and Edith Springs. These sites are very poorly understood, with very little data for them. Surveys undertaken by Gotch (2013a) have mapped some of them as part of the wider GAB spring mapping and they have had their spider fauna sampled (Gotch unpublished data). Other less obvious GDEs include some ephemeral waterholes along creeks and rivers of the Neales catchment, discussed below.
1.1.7.3.5 Lake Eyre Basin surface water dependent aquatic ecosystems
Function of Lake Eyre Basin river systems
In contrast to the GAB and its dependent aquatic ecosystems, the variability, ephemerality and salinity of the surface water systems in the Arckaringa subregion has limited their potential for development, resulting in the hydrology of these systems being largely unaltered. Consequently, all catchments of the LEB have been assessed to be in good health (at the reach scale), based on the available monitoring data (LEBSAP, 2008). The conservation risk of the LEB’s ecosystems have been assessed to be of least concern according to IUCN criteria (Pisanu et al. 2014) and few aquatic species are rated as vulnerable or endangered (Gillam and Urban, 2013). This contrasts to other major drainage systems in Australia, (such as the Murray Darling Basin (MDBC, 2008)), and many international river systems, making the LEB unique nationally and internationally (LEBSAP, 2008). The maintenance and protection of refugia (e.g. waterholes) and processes (such as flooding and connectivity) are critical to ensuring the ongoing persistence of species and ecosystems in arid-zone rivers (Costelloe and Russel, 2014). This will enable species within these systems to persist through the dry phases (resistance) and disperse and rebuild populations during wet periods (resilience) (McNeil et al., 2011a).
The ecological processes occurring in the LEB river systems are driven by highly variable hydrology and climate, and the hydrological and geomorphological processes that determine the range of aquatic ecosystem habitats and the connectivity of habitats. Ecological processes are influenced by high levels of disturbance and variability. To survive in the LEB, species have evolved life strategies that enable them to survive long periods of little to no flow, harsh environmental conditions, and unpredictable flow events (Arthington and Balcombe, 2011). Large floods trigger spectacular booms in biotic production in the LEB (e.g. Kingsford et al., 1999; Balcombe and Arthington, 2009), although the booms in the western catchments are not as spectacular compared with the larger eastern catchments (Reid et al., 2004; Kingsford and Porter, 2008). However, the periods of no flow are as critical in dictating the biotic assemblages that exist in arid environments (Arthington et al., 2005; Rolls et al., 2010). The longer periods of no flow last, the fewer submerged habitats exist and the smaller they become, leading to higher densities of biota, and the more saline and oxygen depleted the remaining habitats become (Arthington and Balcombe, 2011). Species must be able to survive by having desiccation resistant life stages (i.e. survive as eggs or seeds in dry soil), migrating to other areas, becoming temporarily locally extinct and re-populating during the next flow events, or be able to survive in refugia. During extended droughts when obligate aquatic species exist only in Ark refuges, they are completely reliant on those refugia and vulnerable to catchment-wide extinction should the integrity of the refugia be impacted (McNeil et al., 2011a). Local flow events sustain species which are entirely dependent on permanent water by freshening refuges, extending their duration and providing short-term connectivity that enables migration between nearby refugia. While low and no flow phases exert stresses on the biota of aquatic ecosystems, LEB biota have adapted to these unique conditions over millennia of increasingly harsh environmental conditions (Davis et al. 2013).
Because of the extreme fluctuations in flood and drought that drive the boom and bust ecology of the LEB, and associated fluctuations in species populations (Bunn et al., 2006; Kingsford et a., 1999; Balcombe and Arthington, 2009), it is difficult to determine whether ecosystems are healthy or impacted (Sheldon, 2005) and the extinction risk status of species (Costelloe and Russel 2014. During extended droughts when obligate aquatic species exist only in Ark refuges, they are completely reliant on those refugia and vulnerable to catchment-wide extinction should the integrity of the refugia be impacted (Arthington et al., 2005; Costelloe and Russel, 2014). Indicators of ‘condition’ (e.g. species diversity, fish health, riparian vegetation health, water quality) in refuges decline as the waterbody evaporates and animals converge on the waterholes for food and water (Sheldon, 2005; Arthington et al., 2005). When floods occur, the diversity, abundance and health of flora and fauna increases (e.g. Costelloe et al., 2004; Balcombe and Arthington, 2009; Arthington and Balcombe, 2011).
In the LEB, maintaining the health of the rivers and wetlands is an obligation under the LEB Intergovernmental Agreement and therefore assessments of the health of rivers and wetlands are required. However, the extreme spatial and temporal variability found throughout the LEB (Bunn et al., 2006; Kingsford et a., 1999), coupled with data deficiencies and an understanding of the system functions that is still evolving, have provided challenges to developing a method for assessing river health (Sheldon, 2005). Therefore, a Strategic Adaptive Management (SAM) approach has been adopted that incorporates Thresholds of Potential Concern (TPCs) to assess river health (Thoms et al., 2009). The TPCs describe the limits of acceptable change beyond which the systems shift to an ‘undesirable’ state (Thoms et al., 2009) and are therefore suited to systems that are still in a ‘desired’ state. The LEB Rivers Assessment (LEBRA) monitoring program involves annual monitoring of a primary set of primary indicators: fish, water quality, and hydrology (DSEWPAC 2011, 2012, 2013). Within the Arckaringa subregion, the Neales River catchment is the only catchment to be included in the LEBRA. LEBRA monitoring data are summarised in Cockayne et al., (2012, 2013) but has not been interpreted in terms of condition reporting.
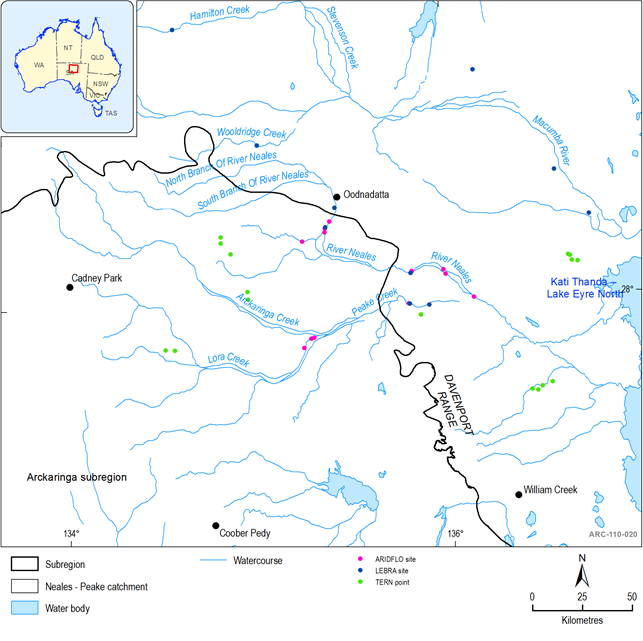
Within the Arckaringa subregion, only the Neales catchment has been the subject of any assessment and monitoring of the aquatic biota through the ARIDFLO project (Costelloe et al., 2004), LEBRA (McNeil et al., 2008, 2009; Cockayne et al., 2012, 2013), (see Figure 42) Critical Refugia Project (McNeil et al., 2011a) and EPA monitoring (Goonan et al., 2003; EPA 2012). There is limited spatial distribution in the data and no time series data for the biota of surface water-driven aquatic ecosystems in other catchments of the Arckaringa subregion.
The LEB Rivers Monitoring (LEBRM) project has been funded by the Australian Government Department of the Environment through the Office of Water Science to improve the level of knowledge about surface water dependent aquatic ecosystems in and near the Arckaringa and Pedirka subregions to inform the Lake Eyre Basin Bioregional Assessment. The focus of this project is on collating existing knowledge; collecting new hydrological, geomorphological and ecological knowledge; and hydrological modelling and hydro-ecological analysis. The LEB Rivers Monitoring project is being undertaken by the South Australian Government Department of Environment, Water and Natural Resource and is scheduled for completion in 2015. The Goyder Institute for Water Research is undertaking an LEB project that builds on the LEBRM and LEBRA projects to develop a suite of indices to inform management decisions and condition monitoring.
Figure 42 Location of aquatic ecosystem monitoring sites
Neales catchment
The Neales catchment is highly ephemeral, with only one known potentially permanent fresh waterhole, Algebuckina Waterhole. Ten native and one introduced fish species (Gambusia holbrooki) have been found in the Neales catchment (Costelloe et al., 2004; McNeil et al., 2011a; Cockayne et al., 2013; Table 23). None of the native species are rated as threatened nationally or at a state level, however all are rated as regionally rare (Gillam and Urban, 2013). These species are a subset of those species found in the eastern catchments and it is assumed that there may have been some migration between catchments across Kati Thanda – Lake Eyre during extremely large simultaneous flood events in the past, but that Kati Thanda – Lake Eyre is generally too salty when it fills for this to occur. Genetic studies are required to determine the relationship between the Neales catchment fish population and populations in other LEB catchments. Current genetic programs being undertaken at Monash and Flinders Universities may provide insight into these patterns of Basin scale connectivity. Distinct assemblages of biotic groups occur in the Neales catchment compared with the larger eastern catchments of the Cooper and Georgina-Diamantina associated with higher ephemerality and higher salinities (Costelloe et al., 2004; Madden et al., 2002). For fish, the community comprises a subset of the more hardy species found in the eastern catchments, however the genetic linkages between populations in separate catchments is currently unknown.
Table 23 Riverine fish fauna found of the Neales catchment
Source: Cockayne et al., 2013 Appendix A, updated from Unmack and Wager (2000). Exotic/introduced species are indicated*, endemic to the LEB are indicated^
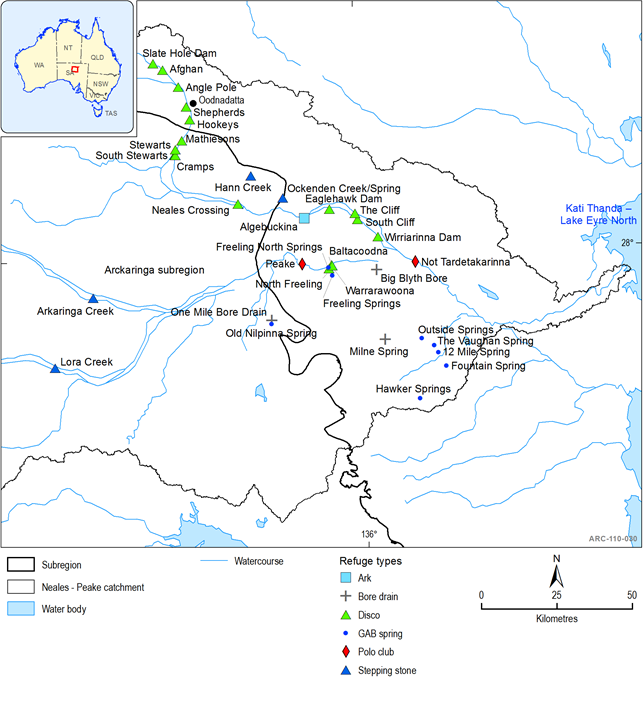
During periods of extended drought conditions, Algebuckina Waterhole supports the entire fish diversity of the Neales catchment (McNeil et al., 2011a, McNeil and Schmarr, 2009), providing an ‘Ark refuge’ (Robson et al., 2008); it is therefore of critical ecological value (Costelloe and Russel 2014) (Figure 43). Water quality monitoring has shown that Algebuckina and Peake Crossing waterholes come under considerable pressure during dry periods, evidenced by nutrient enrichment and low macroinvertebrate numbers (EPA, 2013). When sufficient rainfall allows for in-channel connectivity to occur, fish species radiate out from Algebuckina to recently inundated waterholes. Fish establish populations in progressively distant habitats following predictable patterns of species resilience, with more resilient species establishing first, followed by less resilient species (McNeil and Schmarr, 2009; Kerezsy et al., 2013). Saline waterholes also persist through droughts; however these are only able to support highly tolerant species and fit the description of ‘polo club refuges’ (Robson et al., 2008; McNeil et al., 2011a). In addition, at least two spring-fed pools in the Peake Creek adjacent to Freeling Springs are also likely to serve as permanent refugia for five of the smaller bodied species, but not for the entire riverine community (McNeil et al., 2012). It is probable that stable and shallow bore and spring habitats also serve as key refuges for the pest fish Gambusia holbrooki that, during periods of connectivity, seed the riverine ecosystem with new recruits (McNeil and Costelloe, 2011). It appears, however, that the combination of a natural and variable flow regime and an intact native fish fauna prevent the dominance of Gambusia through the broader riverine ecosystem (Puckridge et al., 2000; McNeil et al., 2012).
Other refuge types within the Neales catchment are ‘disco’ refuges (non-permanent waterholes where fish rebuild populations during extended wet periods) and ‘stepping stones’ – temporary habitats used during migration but insufficient to support populations for any extended period (McNeil et al., 2011a). Many farm dams have been sampled and their fish assemblages are representative of natural disco-type refugia (McNeil et al., 2009), however, their role as refugia is not known.
While the Neales catchment has been found to support a range of waterbird species, the diversity (44 species) and abundance of waterbirds was the lowest of the SA LEB reaches studied in ARIDFLO, probably due to the relatively small area of suitable habitat. However, NPWSA rated bird species were found: Freckled Duck, Brolga (both vulnerable), and Blue-billed Duck (rare) (Reid et al., 2004). Floods in western catchments, including the Neales catchment in 2000, contributed to partial filling of Kati Thanda – Lake Eyre which resulted in a major breeding event for Banded Stilts (Cladorhynchus leucocephalus) (Reid et al., 2004), a vulnerable (NPWSA) migratory waterbird.
Other Lake Eyre Basin catchments
The aquatic ecosystems and their species composition in other catchments of the LEB in the Arckaringa subregion are poorly documented. Spangled Perch (Leiopotherapon unicolor), Desert Goby (Chlamydogobius eremius), and Lake Eyre Hardyhead (Craterocephalus eyresii) have been observed at road crossings in Margaret Creek and Stuarts Creek (McNeil pers. com. 2013; SARDI unpublished data), indicating permanent refugia must exist in this catchment. It is likely that GAB springs on Billa Kallina Station may support permanent pools upstream of the crossing (Lloyd Sampson pers. com., 2013); further survey work is required to confirm if this is the case. There are also GAB springs in close proximity to the watercourses in the Warinner Creek catchment, but nothing is documented about the aquatic ecology of this catchment.
Floodplain and swamp ecosystems are considered to be under threat because their high productivity and presence of water results in heavier grazing pressure by stock and pest animals (DEH & SAAL NRMB 2009).
The endorheic Lake Cadibarrawirracanna catchment is included within the LEB, but is not hydrologically connected with Kati Thanda – Lake Eyre. Nothing is documented about the aquatic ecosystems of this catchment other than from one-off biological surveys; however it is likely that the lake may support biota tolerant of extreme drought and salinity and possibly migratory waterbirds when it occasionally fills.
1.1.7.3.6 Surface water dependent aquatic ecosystems outside the Lake Eyre Basin
In the part of the subregion that is outside the LEB, there are only very minor surface water dependent aquatic ecosystems that are poorly documented. These consist of dune lakes and depressions in the Great Victoria Desert IBRA bioregion and minor drainages and gilgais and cracking clay plains in the Stony Plains IBRA bioregion. These systems only flow or fill very occasionally from local rainfall and are too shallow to hold water for long, however they support some desert fauna such as frogs, lizards and zooplankton. Flora species adapted to survive in these conditions provide resources for fauna species. These lakes are also critical breeding habitat for burrowing frogs and essential habitat for nomadic waterbirds. The cracking clay plains and gilgai systems are a conservation priority in the Stony Plains as they support nationally threatened fauna, the Plains Rat (Pseudomys australis) and Thick-billed Grasswren (Amytornis textilis modestus) (DEH & SAAL NRMB, 2009). There is little hydrological connectivity between the aquatic ecosystems outside the LEB.
Product Finalisation date
- 1.1.1 Bioregion
- 1.1.2 Geography
- 1.1.3 Geology
- 1.1.4 Hydrogeology and groundwater quality
- 1.1.5 Surface water hydrology and surface water quality
- 1.1.6 Surface water – groundwater interactions
- 1.1.7 Ecology
- Citation
- Acknowledgements
- Contributors from the Government of South Australia
- Contributors to the Technical Programme
- About this technical product