- Home
- Assessments
- Bioregional Assessment Program
- Pedirka subregion
- 1.1 Context statement for the Pedirka subregion
- 1.1.7 Ecology
- 1.1.7.3 Aquatic species and communities
The aquatic ecosystems of the Pedirka subregion can be broadly grouped into the groundwater-dependent Great Artesian Basin (GAB) springs and the surface water-dependent ecosystems which lie within the LEB (Figure 43). The following sections describe each of these groupings, firstly in terms of the broader aquatic ecosystems and habitats and secondly in relation to the species and communities therein. The aquatic biota of isolated dune lake systems are very poorly understood and in the Pedirka subregion have not been studied.
Because the GAB springs are decoupled from regional climatic rainfall changes, they provide evolutionary refugia for relict and short-range endemic species that have limited capacity to persist or disperse without permanent water. The GAB spring ecosystems are therefore highly vulnerable because their biota cannot repopulate springs once they become locally extinct. Perennial waterholes in arid-zone river systems provide ecological refuges for obligate aquatic species and are responsive to local and regional climatic conditions (Davis et al., 2013). Within the Pedirka subregion there are no known perennial waterholes; in the Finke catchment perennial waterholes exist upstream of the Pedirka subregion while the Macumba catchment is most likely to be reliant on refuge waterholes in the lower Georgina-Diamantina catchment. Populations of aquatic biota have distinct assemblages and distributions at different scales (e.g. spring vent, spring group, waterhole, and catchment) depending on their dispersal mechanisms and capacity to withstand conditions within those environments. The relationship between hydrological connectivity and dispersal between catchments can only be determined by genetic studies and is a priority for further research (Davis et al., 2013). The long term persistence of the species and communities of the Pedirka subregion aquatic ecosystems is dependent on maintaining the environmental drivers that support the metapopulation dynamics and requirements of biota through connectivity, refuges, resistance and resilience (Davis et al., 2013). Therefore, the discussion below identifies both the species and communities within the aquatic ecosystems as well as the processes and habitat requirements that are critical to their survival.
Using data sourced from the Biological Database of South Australia and Atlas of living Australia, the distribution of threatened fish species for the Pedirka subregion are shown in Figure 39 . All records from sites within a 5 km buffer of the subregion are displayed and shown as having a conservation ranking if they have a threatened status of critically endangered, endangered, vulnerable or rare under Commonwealth and State legislation, together with Regional ratings.
Source: Dalhousie GAB Springs (Fensham et al. 2005) and Northern Territory significant wetland sites (Duguid 2011)
1.1.7.3.1 Fish
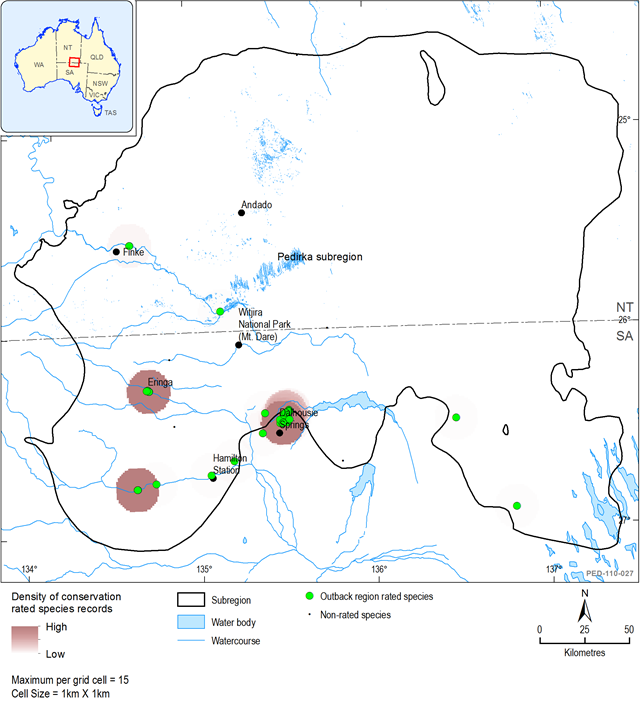
Twelve taxa from 8 families are recorded for the area. None have an EPBC or SA NPWS Act rating, however, regionally 6 are considered rare and 5 vulnerable (Table 11). Figure 44 shows the distribution of rated species records within each rating category and the density of rated species records within 1 km2 grid cells. The highest density of rated species is at Dalhousie Springs and in the Macumba catchment in Hamilton Creek and Lindsay Creek (Figure 44).
Table 11 List of fish with conservation status ratings at the National (EPBC Act), South Australian (SA NPWS Act), or regional level (Outback NRM Region) recorded within the Pedirka subregion
Figure 44 Significant fish species within and near the Pedirka subregion
1.1.7.3.2 Amphibians
Seven taxa from two families have been recorded from the Pedirka subregion (Table 12). The Shoemaker Frog (Neobatrachus sutor) has a state conservation status rating of Vulnerable, with a single record from within the subregion. All the frog taxa have close affinities with drainage lines and floodplains.
Table 12 List of amphibians recorded within the Pedirka subregion
aWetland, drainage-line or floodplain dependent taxa are indicated
1.1.7.3.3 Groundwater-dependent aquatic ecosystems
Function of Great Artesian Basin springs
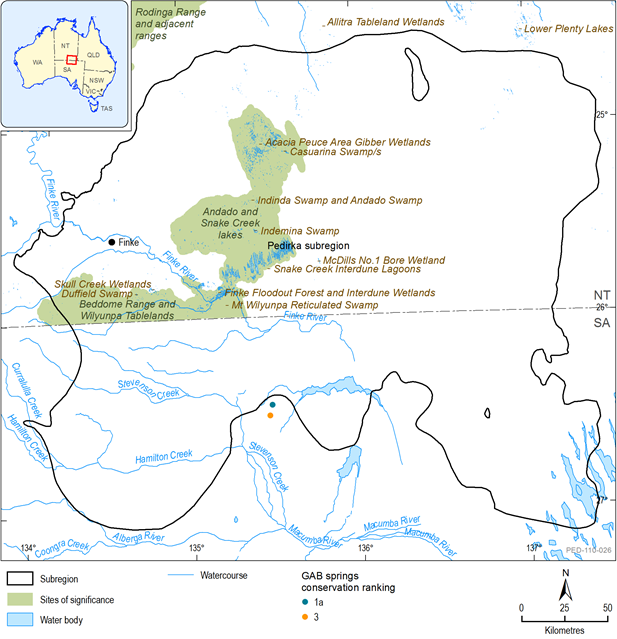
Spring ecosystems dependent on discharge from the GAB are rated as nationally endangered and are protected under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act). The Dalhousie GAB springs occur near the southern boundary of the Pedirka subregion (Figure 43); the hydrogeology in relation to these springs is discussed in Section 1.1.4. The Dalhousie Springs are listed in the Directory of Important Wetlands in Australia, and scored very high in three out of four HEVEA criteria for the LEB, these being distinctiveness, diversity and evolutionary history (Hale 2010). To the north of Pedirka subregion, the GAB flows in an easterly direction towards the Mulligan River supergroup of springs in Queensland (Rousseau-Gueutin et al., 2013).
Gotch (2013a) has developed a spring nomenclature to provide a common language to describe GAB springs in SA (Table 13) based on previous work by Ponder (1986) and Fatchen and Fatchen (1993). A similar nomenclature is adopted in Queensland based on Fensham and Fairfax (2003), however, the latter used distance definitions to separate between spring groups and between spring complexes. The Dalhousie Springs comprise 148 spring vents from 12 spring groups (Gotch, 2013b). The Fensham et al. (2005) dataset separates the springs at Dalhousie into two complexes while Gotch (2013a) identifies all springs as belonging to a single complex. The Dalhousie Springs have the largest outflow of any GAB springs in the arid zone (Habermehl, 1982), with the most recent estimates being between 54 ML/day (Sibenaler, 1996) and 60 ML/day (Gotch, 2013c) using the tool developed by White and Lewis (2011).
The term ‘mound spring’ has historically been used to describe GAB springs, however, not all GAB springs form the characteristic mound. Green et al. (2013) presents a hierarchical classification for GAB springs in SA consistent with the Australian National Aquatic Ecosystems (ANAE) classification framework (AETG 2012a), incorporating the national attributes at the ‘higher levels’. The ‘lower levels’ provide greater differentiation for GAB springs, with attributes for hydraulic environment (artesian or non-artesian), structural linkage to aquifer source and seven surface morphology types (Table 14) (Green et al., 2013). The Dalhousie Springs include carbonate mounds, sand and silt mounds and carbonate terraces (Habermehl, 1982; White et al., 2013b).
Table 13 Spring classification hierarchy and definitions
Source: Gotch (2013a, p. 15, Table 2.2)
Table 14 Different surface morphology types for artesian GAB springs
Source: Green et al. (2013, p. 20)
The Gotch (2013a) survey recorded vent position and elevation to a very high degree of accuracy as well as other baseline information on the condition and ecological values such as flow status, water chemistry, stock damage, and species inventory. Elevation of the spring vent is a vent attribute as it can be subtracted from the source aquifer pressure to determine the groundwater pressure above the spring surface (Green and Berens, 2013). The pressure above spring surface is an indicator of the vulnerability of spring discharge to reduction in groundwater level, with pressure above spring surface of five metres or less indicative of springs at very high risk of ceasing to flow due to reductions in groundwater pressure (Green et al., 2013). Pressure above spring surface has been calculated for the majority of surveyed GAB springs in the artesian zone in SA based on the potentiometric map for the JK aquifer (see Green et al., 2013 pp.29-35).
The area of the wetland has been shown to vary over time both between seasons, mainly driven by the phenology of the dominant vegetation species, and over longer time periods in response to rainfall variation (White et al., 2013a). Some vents within a spring group may be permanently connected, by discharging to the same pool, by overland flow, or by saturated soil conditions. Because of intra- and inter annual variation (White et al., 2013a), vents within a spring group that are not permanently connected may become connected seasonally or ephemerally. Within a spring wetland, there is variation in water chemistry and soil conditions which drives variation in plant species composition (Clarke et al., 2013) and habitats. The flow rate of a spring is one of the main determinants of the total wetland area, the connectivity of the wetland system, the water and soil chemistry (Green et al., 2013), and range of vegetation and habitats (Clarke et al., 2013) present within a wetland. Therefore, the ecological values of GAB springs, including those with a relatively high pressure above the spring vent, can be impacted by relatively small reductions in pressure and hence flow.
GAB springs can range from a small soak to large wetland areas supported by numerous vents. Species diversity has been shown to be positively correlated with the number of vents in a spring group (Ponder, 1986; Clarke et al., 2013). Because the conductivity of each spring vent varies, the rate of flow for any given spring vent cannot be determined from pressure above spring surface alone (Green and Berens, 2013). Spring flow rates are difficult to measure on-ground, however wetland area can be monitored by remote sensing methods as a surrogate for monitoring spring flow rate (White and Lewis, 2011, 2012).
The LEB Springs Assessment project has been funded by the Australian Government Department of the Environment through the Office of Water Science to improve the level of knowledge about GAB springs in and near the Arckaringa and Pedirka subregions to inform the Lake Eyre Basin Bioregional Assessment. The focus of this project is on collating existing knowledge about GAB springs and associated wetland habitats, collecting new hydrogeological and ecological knowledge to address knowledge gaps, and assimilating data into hydrogeological and eco-hydrological modelling. The LEB Springs Assessment is being undertaken by the SA Government Department of Environment, Water and Natural Resource and the Queensland Department of Science, Information, Technology, Innovation and the Arts and is scheduled for completion in 2015.
Dalhousie Springs
The Dalhousie Springs are estimated to have been active for between one and two million years (Krieg, 1989); none have ceased flowing in recent history (Fensham et al., 2010). The Dalhousie Springs are surrounded by extremely arid environments, effectively existing as aquatic islands within a sea of desert. The isolation of springs and their persistence over millions of years of increasingly arid climate change has contributed to high levels of relict and short-range endemic species (Davis et al., 2013; Gotch, 2013b).
Fensham et al. (2010) provides a conservation ranking for spring complexes based on the presence of significant species and condition of the wetland environment (Table 15); the highest rank for an individual spring within a complex is applied to the complex (Fensham et al., 2010). Within Dalhousie supergroup, Dalhousie Springs complex is ranked 1a and Dalhousie Ruins ranked 3 (Fensham et al., 2005). Five species of fish, three crustaceans and three molluscs were identified that are found only at Dalhousie Springs (Fensham et al., 2010) (Table 16). There are, however, limitations to identifying ecological values by endemism and species presence as not all springs have been comprehensively surveyed, and some biotic groups have been better surveyed and classified taxonomically than others. Additionally, the Fensham et al. (2010) ranking is based on data collated in 2005 (Fensham et al., 2005) and additional research (both in field survey and taxonomy) has since been undertaken. Gotch et al. (2008) found very high levels of short-range endemism to area amongst wolf (lycosid) spiders at Dalhousie compared with other GAB springs in SA. A recent survey and genetic analysis of invertebrate fauna of Lake Eyre supergroup springs identified 42 new ‘evolutionarily significant units[1]’, 16 of which were restricted to only one spring group (Guzik and Murphy, 2013). This research indicates it is likely that the diversity of short-range endemic species has probably been vastly under estimated.
Table 15 Conservation ranking criteria for Great Artesian Basin spring complexes
Source: Fensham et al. (2010)
Table 16 Species endemic to Dalhousie Springs
Source: Fensham et al. (2010)
The Dalhousie Springs supports almost 1000 ha of wetlands, of which Common Reed (Phragmites australis) reedbeds are the main vegetation type, followed by White Tea Tree (Melaleuca glomerata) open forests and woodlands which occur mainly around the spring pools (White et al., 2013b). The flora species diversity is very high by comparison with other GAB springs (Gotch, 2013c), with 104 species recorded to date (Noack, 2005) in 44 plant communities (Mollemans, 1989). Many of these species are widespread in more temperate and tropical regions, but are highly restricted within central Australia (Mollemans, 1989; Noack, 2005). 155 bird species, including 56 waterbirds and several migratory species, have been recorded at Dalhousie Springs (Noack, 2005). The wetlands drain into Spring Creek, which runs for approximately 50 km before terminating in the Simpson Desert (Wolaver et al., 2013).
Despite its isolation, the ecology of Dalhousie Springs has been impacted by changes in land management practices. Date Palms (Pheonix dactylifera), which were planted at Dalhousie in the late 1800s for food, have become a major weed that significantly reduces natural flows due to their very high water requirements (Gotch, 2013c). Nearly 2,500 Date Palms were recently removed, but outlying and small plants remain (Gotch, 2013c). Changes in the fish composition of pools, including local extirpations, has been attributed to loss of open water habitat due to vegetation growth resulting from changes in burning and grazing regimes (Kodric-Brown et al., 2007).
Recent studies of aquatic invertebrate fauna in GAB springs in SA have found there is very little dispersal between spring groups and complexes (Guzik and Murphy, 2013). Genetic studies of plant species disjunct from their main range in coastal Australia found they are genetically distinct from their major coastal populations, having become isolated a long time ago (Clarke et al., 2013). Genetic differences between populations at different GAB spring groups also indicated there is limited dispersal of these flora between spring groups (Clarke et al., 2013). Many western GAB springs contain microstromatolites, which are poorly understood (Gotch, 2013b) but are thought to play an important role in the formation of spring mounds (Keppel et al., 2013).
Bore drain wetlands
Bore drain wetland in the Pedirka subregion include McDills No.1 and Purni Bores. McDills No.1 Bore sources water from the GAB and has been rehabilitated to control the rate of flow so that a small area of wetland and associated wildlife are supported, as directed by the Traditional Owners (Duguid, 2011). Bore drain wetland ecosystems have low ecological values, with only a reduced diversity of GAB spring flora that does not include the unique relict and endemic species found in natural GAB spring wetlands (Fensham and Fairfax, 2003; Gotch, 2013b). Some bore drain wetlands are, however, valued by the community, including residents and tourists (Phipps 2008) and some may possibly have been constructed to enhance the flow of pre-existing spring vents that may have held cultural significance (Louise Hercus pers. comm. 2013).
Other groundwater-dependent ecosystems
While it is considered likely that alluvial groundwater may prolong the persistence of some waterholes and support other aquatic ecosystems such as floodplain and floodout woodlands and forests (Duguid 2011), there have been no studies to determine the contribution of alluvial groundwaters in the Pedirka subregion.
1.1.7.3.4 Surface water dependent aquatic ecosystems
Function of Lake Eyre Basin river systems
In contrast to the GAB and its dependent aquatic ecosystems, the variability, ephemerality and salinity of the surface water systems in the Pedirka subregion has limited their potential for development, resulting in the hydrology of these systems being largely unaltered. Consequently, all catchments of the LEB have been assessed to be in good health (at the reach scale), based on the available monitoring data (LEBSAP, 2008). The conservation risk of the LEB’s ecosystems have been assessed to be of least concern according to IUCN criteria (Pisanu et al. 2014) and few aquatic species are rated as vulnerable or endangered (Gillam and Urban, 2013). This is in contrast to other major drainage systems in Australia, (such as the Murray Darling Basin (MDBC, 2008)) and internationally, making the LEB unique nationally and internationally (LEBSAP, 2008). Therefore, it is the maintenance and protection of the components (such as refugia) and processes (such as flooding and connectivity) that enable the species within these systems to persist through the dry phases (resistance) and disperse and rebuild populations during wet periods (resilience) that are critical to ensuring the ongoing persistence of the ecosystems (McNeil et al., 2011a).
The ecological processes occurring in the LEB river systems are driven by highly variable hydrology and climate, and the hydrological and geomorphological processes that determine the range of aquatic ecosystem habitats and the connectivity of habitats. Ecological processes are influenced by high levels of disturbance and variability. To survive in the LEB, species have evolved life strategies that enable them to survive long periods of little to no flow, harsh environmental conditions, and unpredictable flow events (Arthington and Balcombe, 2011). Large floods trigger spectacular booms in biotic production in the LEB (e.g. Kingsford et al., 1999; Balcombe and Arthington, 2009), although the booms in the western catchments are not as spectacular compared with the larger eastern catchments (Reid et al., 2004; Kingsford and Porter, 2008). However, the periods of no flow are as critical in dictating the biotic assemblages that exist in arid environments (Arthington et al., 2005; Rolls et al., 2010). The longer periods of no flow last, the fewer submerged habitats exist and the smaller they become, leading to higher densities of biota, and the more saline and oxygen depleted the remaining habitats become (Arthington and Balcombe, 2011). Species must be able to survive by having desiccation resistant life stages (i.e. survive as eggs or seeds in dry soil), migrating to other areas, become temporarily locally extinct and re-populating during the next flow events, or be able to survive in refugia. During extended droughts when obligate aquatic species exist only in Ark refuges, they are completely reliant on those refugia and vulnerable to catchment-wide extinction should the integrity of the refugia be impacted (McNeil et al., 2011a). Local flow events sustain species which are entirely dependent on permanent water by freshening refuges, extending their duration and providing short term connectivity that enables migration between nearby refugia. While low and no flow phases exert stresses on the biota of aquatic ecosystems, LEB biota have adapted to these unique conditions over millennia of increasingly harsh environmental conditions (Davis et al. 2013).
Because of the extreme fluctuations in flood and drought that drive the boom and bust ecology of the LEB, and associated fluctuations in species populations (Bunn et al., 2006; Kingsford et a., 1999; Balcombe and Arthington, 2009), it is difficult to determine whether ecosystems are healthy or impacted (Sheldon, 2005) and the extinction risk status of species (Costelloe and Russel 2014. During extended droughts when obligate aquatic species exist only in Ark refuges, they are completely reliant on those refugia and vulnerable to catchment-wide extinction should the integrity of the refugia be impacted (Arthington et al., 2005; Costelloe and Russel, 2014). Indicators of ‘condition’ (e.g. species diversity, fish health, riparian vegetation health, water quality) in refuges decline as the waterbody evaporates and animals converge on the waterholes for food and water (Sheldon, 2005; Arthington et al., 2005). When floods occur, the diversity, abundance and health of flora and fauna increases (e.g. Costelloe et al., 2004; Balcombe and Arthington, 2009; Arthington and Balcombe, 2011).
In the LEB, maintaining the health of the rivers and wetlands is an obligation under the LEB Intergovernmental Agreement and therefore assessments of the health of rivers and wetlands are required. However, the extreme spatial and temporal variability found throughout the LEB, coupled with data deficiencies and an understanding of the system functions that is still evolving, have provided challenges to developing a method for assessing river health. Therefore, a Strategic Adaptive Management (SAM) approach has been adopted that incorporates Thresholds of Potential Concern (TPCs) to assess river health (Thoms et al., 2009). The TPCs describe the limits of acceptable change beyond which the systems shift to an ‘undesirable’ state (Thoms et al., 2009) and are therefore suited to systems that are still in a ‘desired’ state. The LEB Rivers Assessment (LEBRA) monitoring program involves annual monitoring of key parameters: fish, water quality, and hydrology (DSEWPAC, 2011, 2012, 2013). LEBRA monitoring data are summarised in Cockayne et al., (2012, 2013) but has not been interpreted in terms of condition reporting.
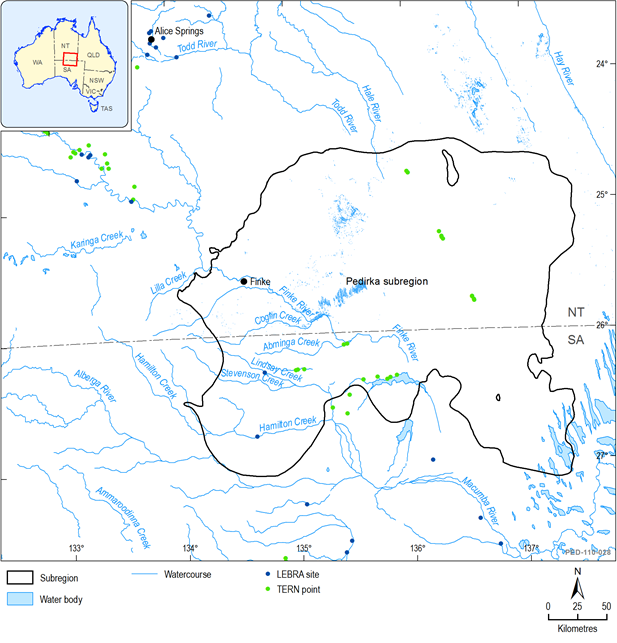
Within the Pedirka subregion, the Macumba and Finke catchments have been monitored for the LEBRA since autumn 2011, however all LEBRA sites in the Finke catchment are located upstream of the Pedirka subregion (Cockayne, 2012, 2013) (Figure 45). Sites within the Macumba catchment have also been monitored by the SA EPA with monitoring including macroinvertebrates and water quality parameters (monitoring has been twice, ten years apart; Goonan et al., 2003; EPA, 2012). While there have been some additional one-off biological surveys (most notably the SA Arid Rivers biological survey 2005/2006, and surveys by Duguid 2011 and Eldridge and Reid 1998), the Pedirka subregion contains some of the least studied aquatic ecosystems in the LEB; in particular, the dune lakes in the south-eastern part of the subregion that have not been the subject of any studies (e.g. Lakes Peera Peera Poolana and Griselda).
The LEB Rivers Monitoring (LEBRM) project has been funded by the Australian Government Department of the Environment through the Office of Water Science to improve the level of knowledge about surface water dependent aquatic ecosystems in and near the Arckaringa and Pedirka subregions to inform the Lake Eyre Basin Bioregional Assessment. The focus of this project is on collating existing knowledge; collecting new hydrological, geomorphological and ecological knowledge; and hydrological modelling and hydro-ecological analysis. The LEB Rivers Monitoring project is being undertaken by the SA Government Department of Environment, Water and Natural Resource and is scheduled for completion in 2015. The Goyder Institute for Water Research is undertaking an LEB project that builds on the LEBRM and LEBRA projects to develop a suite of indices to inform management decisions and condition monitoring.
Figure 45 Location of aquatic ecosystem monitoring sites
Table 17 Riverine fish fauna found in the Macumba and Finke catchments
Source: Cockayne et al., 2013 Appendix A, updated from Unmack and Wager (2000)
Note: *exotic/introduced, ^endemic to the LEB
Macumba catchment
The Macumba catchment, of which the Stevenson and Hamilton creek subcatchments lie within the Pedirka subregion, is the only surface drainage system within the Pedirka subregion to contribute flow to Kati Thanda – Lake Eyre under current climatic conditions. This it does via outflow into the Kallakoopah Creek, an anabranch of the Warburton Creek in the lower Georgina-Diamantina catchment (see Section 1.1.5). There are no known permanent waterholes in the Macumba catchment, however there are several waterholes that can hold water for over a year without flow. Nine native fish species have been recorded in the catchment, all of which are also found in the Georgina-Diamantina catchment (refer Table 12) (Cockayne et al., 2013). The fish assemblage differs from neighbouring Neales and Finke catchments, indicating the fish of the Macumba catchment are most likely to be sourced from the Georgina-Diamantina catchment rather than via Kati Thanda – Lake Eyre or connectivity with the Finke catchment. The nearest permanent waterhole to the Macumba River-Kalakoopah Creek confluence is Pandie Pandie waterhole, at least 300 to 400 km upstream in Goyders Lagoon.
This biological data supports the role of the Macumba River as a lowland tributary of the Warburton Creek (and therefore the Georgina-Diamantina catchment). However, as both tributary and main stream are highly ephemeral at the point of juncture, both catchments would have to have sufficient synchronous flow of sufficient magnitude and duration for hydrological connectivity to occur long enough for fish dispersal. The parameters of such an event are unknown.
The pattern of fish species dispersal observed in three years of LEBRA monitoring (Cockayne et al., 2012, 2013) is consistent with migration from refugia in the Georgina-Diamantina catchment. LEBRA fish monitoring has shown that species that possess rapid dispersal and colonisation traits (McNeil et al., 2011; Kerezsy et al., 2013) were found to rapidly recolonise the upper reaches of the Macumba catchment following extended connection with the Warburton Creek in 2010. Species with poorer dispersal traits were only recolonised in the lower reaches of the Macumba River, nearer to refuge sources in the Warburton. A similar pattern was observed in the neighbouring Eyre Creek catchment where fish dispersed from refuges in the Georgina-Diamantina catchment into the Mulligan River up to 300 km from the nearest permanent waterhole (Kerezsy et al., 2013). Similar trait-based dispersal patterns were observed with some species dispersing earlier and further while others dispersed later and did not move past deeper waterholes in the middle reaches, or move into the catchment at all (Kerezsy et al., 2013). A similar pattern has also been found in the Neales during recovery from drought (McNeil et al., 2008, 2009, 2011).
While the Macumba catchment is clearly reliant on source populations in the Georgina-Diamantina, it is likely that, if successive floods occur in both catchments before the Macumba catchment waterholes dry out, fish from the Macumba catchment will disperse back into the Georgina-Diamantina catchment. Thus the Macumba catchment may act as ‘nursery’ and feeding area for the ‘parent populations’, similar to the way in which floodplain ecosystems support fish populations during floods (Balcombe et al., 2007), and contribute to the genetic resistance and overall population resilience of the parent populations (McNeil pers. com., 2013). The patterns of hydrological connectivity and availability of emigrational pathways for fish through Kati Thanda – Lake Eyre during simultaneous high flow events in multiple catchments are unknown, however there is potential for connectivity across the Macumba, Warburton, Cooper and Neales catchments during periods of very high rainfall and flow. Current genetic programs being undertaken at Monash and Flinders Universities may provide insight into these patterns of Basin-scale connectivity.
Finke catchment
North of the Macumba catchment, the lower Finke River floods out in the Simpson Desert dunefields within the Pedirka subregion. It has not been known to flow to Kati Thanda – Lake Eyre under current climatic conditions, but is likely to have between 1,000 and 20,000 years ago (Unmack, 2001a) and possibly more recently during occasional mega floods (Pickup, 1991). The Finke catchment contains numerous permanent, seasonal and ephemeral waterholes (Duguid, 2013) however all known permanent waterholes are located upstream of the Pedirka subregion (Duguid, 2011). Within the lower Finke, some waterholes can last over a year without flow, but will dry out without any subsequent flow. Large interdune lakes and pans as well as wooded swamps also occur in the Finke floodout but only fill infrequently and are dry most of the time (Duguid, 2011).
Nine native fish species occur in the Finke, of which three are endemic to that catchment and the remainder are found in other parts of the LEB (Table 17). No surveys have been undertaken in the lower reaches, but fish and piscivorous waterbirds have been observed in the Finke floodout (Duguid, 2011). Nothing is known about the fate of fish in floodouts, but it is likely that they may feed and breed and, if consecutive floods occur, may migrate back upstream. Therefore, these lower reaches may provide an occasional nursery and feeding area for the Finke fish populations. However, the influence of these on populations further upstream may be small and infrequent.
Water-dependent ecosystems are poorly mapped within the Pedirka subregion. Barnetson and Duguid (2010a) undertook some mapping of the LEB within NT and further work was undertaken for the GAB Water Control District (Duguid, 2011), including aerial videography. However, much of the data from the latter is yet to be digitised. Duguid (2011) includes descriptions of selected wetlands and wetland aggregations and compiled survey and anecdotal waterbird data to identify floodout systems known to and likely to support waterbirds. An earlier study of the vegetation, birds and terrestrial vertebrates found the floodouts of the Finke catchment support a range of species of biological significance, including Plains Rat (Pseudomys australis), Thick-billed Grasswren (Amytornis textilis), both classified as Vulnerable to extinction nationally, and Mongolian Plover (Charadrius mongolus), which is rarely recorded inland (Eldridge and Reid 1998). The study found that the Finke floodout provides refuge for terrestrial biota through drought periods (Eldridge and Reid, 1998). There are significant areas of forests and woodlands (both Eucalyptus camaldulensis and Eucalyptus coolabah ssp. arida ) along channels, rivers and swamps that are likely supported by perched watertables (Duguid, 2011; see Section 1.1.6). Duguid (2011) identified three wetlands that meet the criteria of national significance in the Directory of Important Wetlands in Australia, four regionally significant wetlands, and proposed an additional three sites of possible significance for further investigation for the NT part of the Pedirka subregion (Table 18; Figure 43). Andando and Snake Creek Lakes and Beddome Range and Wilyunpa Tablelands are also identified as regions of conservation significance for the NT (Harrison et al., 2009; Ward and Harrison, 2009). Most of the wetlands are isolated from the major river drainages, including many of the ‘significant’ ones. Temporary perched watertables are thought to sustain some wetlands in floodouts for longer periods than rainfall or river flow alone, notably in the Snake Creek interdune wetlands which are part of the Finke floodout (Duguid, 2011).
There are severe infestations of Athel Pine (Tamarix aphylla), a weed of national significance, as well as other moisture-loving weeds, in some aquatic ecosystems of the Finke catchment and other catchments (Duguid pers. com., 2013). These compromise the ‘naturalness’ of these systems, altering the habitats and resources for fauna and outcompeting other native plants. They may also impact the hydrology-geomorphology relationships and alter flow paths.
Isolated surface water systems
The Pedirka subregion also includes many wetlands in the claypans between dunes filled from local rainfall events and not river flow. In the south-east of the Pedirka subregion there are numerous interdune lakes, known as the Peera Peera Poorana and Poeppel Lakes systems, that are fed by direct local rainfall. Some of the more southerly lakes connect with the Kallakoopah Creek (Nanson, 2010 in Hale et al., 2010). Nanson (in Hale et al., 2010) notes that this is the largest complex of dry lakes and interdunes in Australia, but it has been subject to almost no research due to its remoteness and difficulty of access.
Table 18 Potential Directory of Significant Wetlands Sites for NT-GAB, occurring partly or wholly within the Pedirka subregion
Source: Duguid et al. (2005) as presented in Duguid, (2011 p. 26) and Duguid (2011 p. 27)
Product Finalisation date
- 1.1.1 Bioregion
- 1.1.2 Geography
- 1.1.3 Geology
- 1.1.4 Hydrogeology and groundwater quality
- 1.1.5 Surface water hydrology and surface water quality
- 1.1.6 Surface water – groundwater interactions
- 1.1.7 Ecology
- Citation
- Acknowledgements
- Contributors from the Government of South Australia
- Contributors to the Technical Programme
- About this technical product